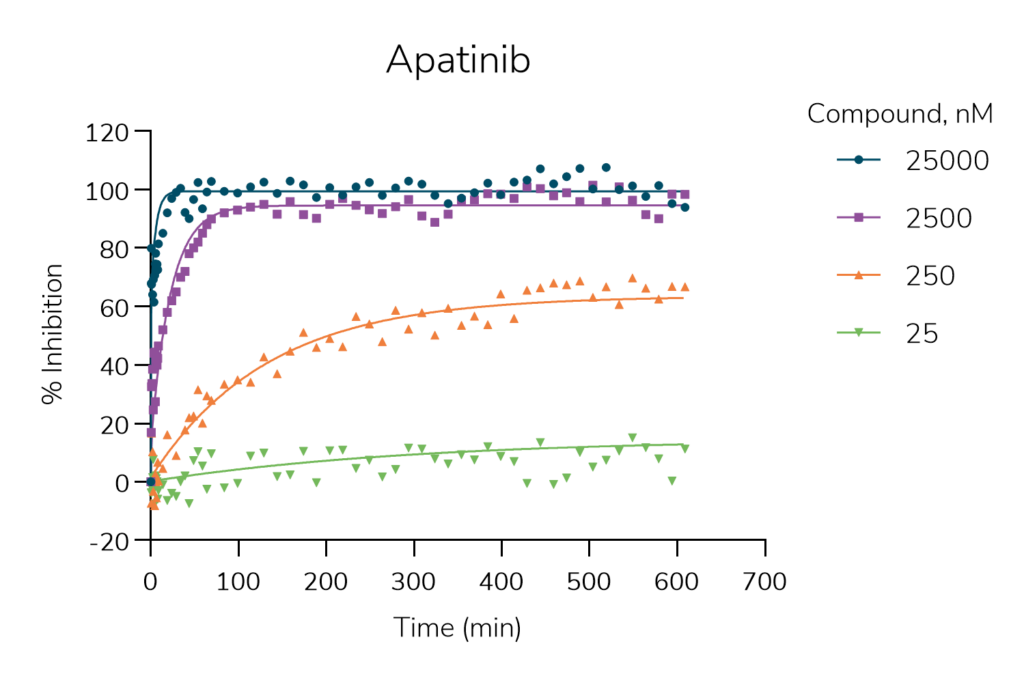KINETICfinder® is the ideal tool for quick prediction of drug clinical efficacy
A clear understanding of binding kinetics provides a more complete picture of what drives target occupancy in vivo and ultimately clinical efficacy: the faster a drug binds to the target, the faster the occupation will occur. Conversely, the longer the duration of binding, the longer the pharmacodynamic effect.
Using KINETICfinder® in the early stages of drug discovery:
- Enables the early identification of clinical effective compounds.
- Allows differentiation between similar therapies.
Approved KIT inhibitors show longer residence time
Many approved kinase inhibitors target KIT kinase, an oncogene implicated in cancer cell growth, proliferation, invasion and metastasis. Figure 1 shows the kinetic profile of 10 approved drugs along with 6 clinical and preclinical compounds against KIT using KINETICfinder®.

Figure 2 and 3. Kinetic curves for two representative KIT inhibitors obtained with KINETICfinder®.
The kinetic plot and Table 1 reveal that longer residence time contributes to the clinical success of inhibitors that target KIT kinase:
- 70% of the clinically efficacious drugs show a long residence time, with a median of 100 minutes, compared to 16% of the clinical and preclinical compounds.
- Development compounds dissociate 13 times faster than marketed drugs, with a median of 8 minutes.
- Approved drugs associate slightly faster to KIT compared to development compounds.
Our results are in agreement with those shown by Georgi et al. in a survey of binding kinetics for 270 compounds targeting 40 clinically relevant kinases. They found that kon values were nearly unchanged between preclinical and approved drugs while koff values shifted toward longer residence time for approved drugs1.
| Kinetic characteristics | Approved drugs | Development drugs |
| Association rate (M-1s-1) | 1.6×105 | 4.5×104 |
| Residence time (min) | 100 | 8 |
| Affinity (nM) | 2.5 | 25 |
| Rapid associating drugs (≥1×106 M-1s-1) | 20% | 16% |
| Slow dissociating drugs (≥1 h) | 70% | 16% |
References
- Georgi V. et al (2018) Binding kinetics survey of the drugged kinome. J Am Chem Soc. 140(46):15774–15782.